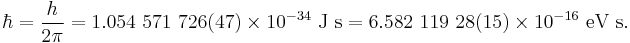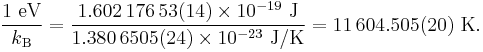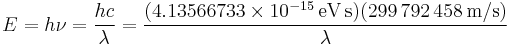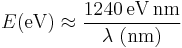Electronvolt
In physics, the electron volt (symbol eV; also written electronvolt[1][2]) is a unit of energy equal to approximately 1.602×10−19 joule (symbol J). By definition, it is the amount of energy gained by the charge of a single electron moved across an electric potential difference of one volt. Thus it is 1 volt (1 joule per coulomb, 1 J/C) multiplied by the electron charge (1 e, or 1.602176565(35)×10−19 C). Therefore, one electron volt is equal to 1.602176565(35)×10−19 J.[3] Historically, the electron volt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences because a particle with charge q has an energy E=qV after passing through the potential V; if q is quoted in integer units of the elementary charge and the terminal bias in volts, one gets an energy in eV.
The electron volt is not an SI unit and its value must be obtained experimentally.[4] Like the elementary charge on which it is based, it is not an independent quantity but is equal to (1 J/C)(2 h α / μ0 c0)0.5 It is a common unit of energy within physics, widely used in solid state, atomic, nuclear, and particle physics. It is commonly used with the SI prefixes milli-, kilo-, mega-, giga-, tera-, or peta- (meV, keV, MeV, GeV, TeV and PeV respectively). Thus meV stands for milli-electron volt.
Atomic properties like the ionization energy are often quoted in electron volts.
In chemistry, it is often useful to have the molar equivalent, that is the energy that would be produced by one mole of charge (6.02214129(27)×1023) passing through a potential difference of one volt. This is equal to 96.4853365(21) kJ/mol.[3]
Contents |
Energy
Conversion factors:
- 1 eV = 1.602176487(40)×10−19 J (the conversion factor is numerically equal to the elementary charge expressed in coulombs).
- 1 eV (per atom) is 96.4853365(21) kJ/mol.[3]
For comparison:
- 5.25×1032 eV: Total energy released from a 20 kT Nuclear Fission Device.
- ~624 EeV (6.24×1020 eV): energy needed to power a single 100 watt light bulb for one second. (100W = 100J/s = ~6.24×1020 eV/s).
- 300 EeV (3×1020 eV) = (50 J) :[5] the so-called Oh-My-God particle (the most energetic cosmic ray particle ever observed).
- 14 TeV: the designed proton collision energy at the Large Hadron Collider (which has operated at half of this energy since 30 March 2010[update]).
- 1 TeV: A trillion electronvolts, or 1.602×10−7 J, about the kinetic energy of a flying mosquito.[6]
- 210 MeV: The average energy released in fission of one Pu-239 atom.
- 200 MeV: The average energy released in nuclear fission of one U-235 atom .
- 17.6 MeV: The average energy released in the fusion of deuterium and tritium to form He-4; this is 0.41 PJ per kilogram of product produced.
- 1 MeV: Or, 1.602×10−13 J, about twice the rest mass-energy of an electron.
- 13.6 eV: The energy required to ionize atomic hydrogen. Molecular bond energies are on the order of one eV per molecule.
- 1.6 to 3.4 eV: the photon energy of visible light.
- 1/40 eV: The thermal energy at room temperature. A single molecule in the air has an average kinetic energy 3/80 eV.
In some older documents, and in the name Bevatron, the symbol BeV is used, which stands for billion electron volts; it is equivalent to the GeV.
Momentum
In high-energy physics, electron-volt is often used as a unit of momentum. A potential difference of 1 volt causes an electron to gain a discrete amount of energy (i.e., 1 eV). This gives rise to usage of eV (and keV, MeV, GeV or TeV) as units of momentum, for the energy supplied results in acceleration of the particle.
The dimensions of momentum units are M 1 L 1 T -1 . The dimensions of energy units are M 1 L 2 T -2 . Then, dividing the units of energy (such as eV) by a fundamental constant that has units of velocity (M 0 L 1 T -1 ), facilitates the required conversion of using energy units to describe momentum. In the field of high-energy particle physics, the fundamental velocity unit is the speed of light c. Thus, dividing energy in eV by the speed of light in vacuum, one can describe the momentum of an electron in units of eV/c.[7] [8]
The fundamental velocity constant c is often dropped from the units of momentum by way of defining units of length such that the value of c is unity. For example, if the momentum p of an electron is said to be 1 GeV, then the conversion to MKS can be achieved by:
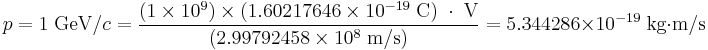
Mass
By mass-energy equivalence, the electron volt is also a unit of mass. It is common in particle physics, where mass and energy are often interchanged, to express mass in units of eV/c2, where c is the speed of light in a vacuum (from E = mc2). It is often common to simply express mass in term of "eV" as a unit of mass, effectively using a system of natural units with c set to 1 (hence, E = m).
For example, an electron and a positron, each with a mass of 0.511 MeV/c2, can annihilate to yield 1.022 MeV of energy. The proton has a mass of 0.938 GeV/c2 (and, in general, the masses of all hadrons are of the order of 1 GeV/c2), which makes the GeV (gigaelectronvolt) a very convenient unit of mass for particle physics:
-
- 1 GeV/c2 = 1.783×10−27 kg
The atomic mass unit, 1 gram divided by Avogadro's number, is almost the mass of a hydrogen atom, which is mostly the mass of the proton. To convert to megaelectronvolts, use the formula:
-
- 1 amu = 931.46 MeV/c2 = 0.93146 GeV/c2
- 1 MeV/c2 = 1.074×10−3 amu
Distance
In particle physics, a system of units in which the speed of light in a vacuum c and the reduced Planck constant ħ are dimensionless and equal to unity is widely used: c = ħ = 1. In these units, both distances and times are expressed in inverse energy units (while energy and mass are expressed in the same units, see Mass–energy equivalence). In particular, particle scattering lengths are often presented in units of inverse particle masses.
Outside this system of units, the conversion factors between electronvolt, second, and nanometer are the following:[3]
The above relations also allow expressing the mean lifetime τ of an unstable particle (in seconds) in terms of its decay width Γ (in eV) via Γ = ħ/τ. For example, the B0 meson has a lifetime of 1.530(9) picoseconds, mean decay length is cτ = 459.7 µm, or a decay width of 4.302±25×10−4 eV.
Conversely, the tiny meson mass mass differences responsible for meson oscillations are often expressed in the more convenient inverse picoseconds.
Temperature
In certain fields, such as plasma physics, it is convenient to use the electronvolt as a unit of temperature. The conversion to kelvins (symbol: uppercase K) is defined by using kB, the Boltzmann constant:
For example, a typical magnetic confinement fusion plasma is 15 keV, or 170 megakelvins.
Properties
The energy E, frequency v, and wavelength λ of a photon are related by
where h is the Planck constant, c is the speed of light. For quick calculations, this reduces to
A photon with a wavelength of 532 nm (green light) would have an energy of approximately 2.33 eV. Similarly, 1 eV would correspond to an infrared photon of wavelength 1240 nm, and so on.
Scattering experiments
In a low-energy nuclear scattering experiment, it is conventional to refer to the nuclear recoil energy in units of eVr, keVr, etc. This distinguishes the nuclear recoil energy from the "electron equivalent" recoil energy (eVee, keVee, etc.) measured by scintillation light. For example, the yield of a phototube is measured in phe/keVee (photoelectrons per keV electron-equivalent energy). The relationship between eV, eVr, and eVee depends on the medium the scattering takes place in, and must be established empirically for each material.
See also
References
- ^ IUPAC Gold Book, p. 75
- ^ SI brochure, Sec. 4.1 Table 7
- ^ a b c d http://physics.nist.gov/constants
- ^ http://physics.nist.gov/cuu/Units/outside.html
- ^ Open Questions in Physics. German Electron-Synchrotron. A Research Centre of the Helmholtz Association. Updated March 2006 by JCB. Original by John Baez.
- ^ CERN - Glossary
- ^ "Units in particle physics". Associate Teacher Institute Toolkit. Fermilab. 22 March 2002. http://quarknet.fnal.gov/toolkits/ati/whatgevs.html. Retrieved 13 February 2011.
- ^ "Special Relativity". Virtual Visitor Center. SLAC. 15 June 2009. http://www2.slac.stanford.edu/vvc/theory/relativity.html. Retrieved 13 February 2011.
External links
- BIPM's definition of the electronvolt
- http://physics.nist.gov/cuu/Constants physical constants reference; CODATA data
|
||||||||||||||||||||